Uso potencial de acetogeninas como coadyuvante en el tratamiento del tumor venéreo transmisible canino. Reporte de caso
Resumen
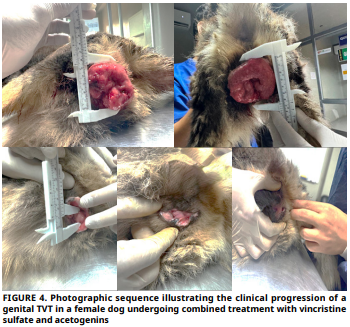
La vincristina, un alcaloide antimicrotubular, es el tratamiento estándar para el tumor venéreo transmisible en caninos debido a su elevada tasa de remisión clínica; sin embargo, recientes investigaciones han explorado su combinación con compuestos naturales bioactivos como estrategia para mejorar la eficacia terapéutica y reducir efectos adversos. El presente estudio evaluó la eficacia clínica de la vincristina en combinación con acetogeninas, fitoquímicos derivados de hojas de Annona muricata, en dos perras con diagnóstico citológico confirmado de tumor venéreo transmisible genital. Ambas pacientes recibieron Vincristina a 0,5 mg·m-² por vía intravenosa, una vez por semana durante seis semanas. En una de las pacientes, desde la primera sesión de quimioterapia, se administró una dosis diaria de 7,5 a 10 mg de acetogeninas durante los primeros 15 días. A partir de la tercera aplicación, la dosis se incrementó a 14,5 a 20 mg·día-1 de acetogeninas, distribuida en dos tomas (una cápsula en la mañana y otra en la noche) junto con la alimentación, y se mantuvo hasta completar cuatro meses de tratamiento. La hembra canina que recibió la terapia combinada presentó una regresión tumoral temprana, con una reducción significativa desde la segunda semana y una remisión clínica del 99,85 % al finalizar el protocolo. No se observaron efectos adversos atribuibles a las acetogeninas durante su administración. En contraste, la paciente tratada únicamente con vincristina mostró una respuesta parcial, con persistencia del tejido tumoral. La combinación de vincristina con acetogeninas se perfila como una estrategia terapéutica prometedora que podría mejorar la eficacia del tratamiento convencional del tumor venéreo transmisible canino. No obstante, estos resultados deben ser validados mediante estudios clínicos controlados, con mayor número de unidades experimentales, evaluaciones histopatológicas y de marcadores moleculares que permitan esclarecer los mecanismos de acción implicados y establecer protocolos estandarizados.
Descargas
Citas
Pimentel PAB, Giuliano A, Odatzoglou P, Ignatenko N, Wenceslau RR, Almeida IO, da Silva PHS, Costa MP, Horta RDS. Clinical guidelines for canine transmissible venereal tumour treatment: systematic review and meta–analysis. Vet. Comp. Oncol. [Internet]. 2025; 23(2):125-140. doi: https://doi.org/pprn DOI: https://doi.org/10.1111/vco.13038
Costa TS, Paiva FN, Manier BSML, Araújo DC, Ribeiro GB, Fernandes JI. Epidemiological, clinical, and therapeutic aspects of canine transmissible venereal tumor in Rio de Janeiro, Brazil (2015-2020). Pesq. Vet. Bras. [Internet]. 2023; 43:e07189. doi: https://doi.org/p3wj DOI: https://doi.org/10.1590/1678-5150-pvb-7189
Franco–Molina MA, Santamaría–Martínez EA, Santana Krimskaya SE, Zarate–Triviño DG, Kawas JR, Ramos–Zayas Y, Palacios–Estrada N, Prado–García H, García–Coronado PL, Rodríguez–Padilla C. In vitro chemosensitivity of a canine tumor venereal transmissible cancer cell line. Front. Vet. Sci. [Internet]. 2022; 9:972185. doi: https://doi.org/p3wk DOI: https://doi.org/10.3389/fvets.2022.972185
Hantrakul S, Klangkaew N, Kunakornsawat S, Tansatit T, Poapolathep A, Kumagai S, Poapolathep S. Clinical pharmacokinetics and effects of vincristine sulfate in dogs with transmissible venereal tumor (TVT). J. Vet. Med. Sci. [Internet]. 2014; 76(12):1549–1553. doi: https://doi.org/f6vksm DOI: https://doi.org/10.1292/jvms.14-0180
Paranzini CS, Sant’anna MC, di Santis GW, Martins MIM. Prevalence of different cytomorphological types of transmissible venereal tumours and the association with prognosis in dogs treated with vincristine sulphate. Retrospective study. Semina Cienc. Agrar. [Internet]. 2015; 36(6):3795-3800. doi: https://doi.org/p3wm DOI: https://doi.org/10.5433/1679-0359.2015v36n6p3795
Antonov A. Successful treatment of canine transmissible venereal tumor using vincristine sulfate. Adv. Res. [Internet]. 2015; 5(5):1-5. doi: https://doi.org/p3wn DOI: https://doi.org/10.9734/AIR/2015/20017
Leil AZA, El–Hallawany HA, Abd El–Rahman HMA. Clinical response of dogs affected with transmissible venereal tumor (TVT) to the chemotherapeutic regime with regard to cytomorphology and histopathology. J. Appl. Vet. Sci. [Internet]. 2022; 7(2):58-65. doi: https://doi.org/p3wp DOI: https://doi.org/10.21608/javs.2022.123900.1132
Sztukowski KE, Yaufman Z, Cook MR, Aarnes TK, Husbands BD. Vincristine–induced adverse events related to body weight in dogs treated for lymphoma. J. Vet. Intern. Med. [Internet]. 2024; 38(3): 1686-1692. doi: https://doi.org/p3wq DOI: https://doi.org/10.1111/jvim.17063
Mealey KL, Fidel J, Gay JM, Impellizeri JA, Clifford CA, Bergman PJ. ABCB1-1Δ polymorphism can predict hematologic toxicity in dogs treated with vincristine. J. Vet. Intern. Med. [Internet]. 2008; 22(4):825-1073. doi: https://doi.org/dzz5n9 DOI: https://doi.org/10.1111/j.1939-1676.2008.0122.x
Poirier M, Blong AE, Walton RAL. Successful management and recovery of a dog with immune–mediated thrombocytopenia following vincristine overdose. J. Vet. Emerg. Crit. Care [Internet]. 2023; 33(4): 539-544. doi: https://doi.org/p3wr DOI: https://doi.org/10.1111/vec.13187
Mason SL, Grant IA, Elliott J, Cripps P, Blackwood L. Gastrointestinal toxicity after vincristine or cyclophosphamide administered with or without maropitant in dogs: a prospective randomised controlled study. J. Small Anim. Pract. [Internet]. 2014; 55(8):391–398. doi: https://doi.org/f6ckbt DOI: https://doi.org/10.1111/jsap.12237
Tsukamoto A, Ohno K, Tsukagoshi T, Maeda S, Nakashima K, Fukushima K, Fujino Y, Takeuchi A, Tsujimoto H. Ultrasonographic evaluation of vincristine–induced gastric hypomotility and the prokinetic effect of mosapride in dogs. J. Vet. Intern. Med. [Internet]. 2011; 25(6):1195-1515. doi: https://doi.org/b5bg35 DOI: https://doi.org/10.1111/j.1939-1676.2011.00795.x
Kelly B, Thamm D, Rosengren RJ. Utility of the second– generation curcumin analogue RL71 in canine histiocytic sarcoma. Vet. Res. Commun. [Internet]. 2024; 48(1):563–568. doi: https://doi.org/p3ws DOI: https://doi.org/10.1007/s11259-023-10201-2
Çetinkaya S, Çınar Ayan İ, Dursun HG, Süntar İ, Taban K, Gök HN, Atak M. Enhanced apoptosis in pancreatic cancer cells through thymoquinone–rich Nigella sativa L. methanol extract: targeting NRF2/HO-1 and TNF–α pathways. Anticancer Agents Med. Chem. [Internet]. 2025; 25(20):1607-1621. doi: https://doi.org/p3wt DOI: https://doi.org/10.2174/0118715206370057250421061226
Ansary J, Giampieri F, Forbes–Hernandez TY, Regolo L, Quinzi D, Gracia–Villar S, Garcia–Villena E, Tutusaus–Pifarre K, Alvarez–Suarez JM, Battino M, Cianciosi D. Nutritional value and preventive role of Nigella sativa L. and its main component thymoquinone in cancer: an evidenced–based review of preclinical and clinical studies. Molecules [Internet]. 2021; 26(8):2108. doi: https://doi.org/gpggt8 DOI: https://doi.org/10.3390/molecules26082108
Fallon–Adido HE, Silva–Chagas CK, Ferreira GG, Carmo–Bastos ML, Dolabela MF. In silico studies on cytotoxicity and antitumoral activity of acetogenins from Annona muricata L. Front. Chem. [Internet]. 2023; 11:1316779. doi: https://doi.org/p3ww DOI: https://doi.org/10.3389/fchem.2023.1316779
Olas B. The antioxidant potential of graviola and its potential medicinal application. Nutrients [Internet]. 2023; 15(2):402. doi: https://doi.org/p3wx DOI: https://doi.org/10.3390/nu15020402
Rady I, Bloch MB, Chamcheu RCN, Banang–Mbeumi S, Anwar MR, Mohamed H, Babatunde AS, Kuiate JR, Noubissi FK, El Sayed KA, Whitfield GK, Chamcheu JC. Anticancer properties of graviola (Annona muricata): a comprehensive mechanistic review. Oxid. Med. Cell. Longev. [Internet]. 2018; 1826170:1-39. doi: https://doi.org/p3wz DOI: https://doi.org/10.1155/2018/1826170
Qazi AK, Siddiqui JA, Jahan R, Chaudhary S, Walker LA, Sayed Z, Jones DT, Batra SK, Macha MA. Emerging therapeutic potential of graviola and its constituents in cancers. Carcinogenesis [Internet]. 2018; 39(4):522–533. doi: https://doi.org/gdbcsw DOI: https://doi.org/10.1093/carcin/bgy024
Manoharan JP, Palanisamy H, Vidyalakshmi S. Overcoming multi drug resistance mediated by ABC transporters by a novel acetogenin – annonacin from Annona muricata L. J. Ethnopharmacol. [Internet]. 2024; 322:117598. doi: https://doi.org/p3w2 DOI: https://doi.org/10.1016/j.jep.2023.117598
Periyasamy L, Muruganantham B, Deivasigamani M, Lakshmanan H, Muthusami S. Acetogenin extracted from Annona muricata prevented the actions of EGF in PA-1 ovarian cancer cells. Protein Pept Lett. [Internet]. 2021; 28(3):304–314. doi: https://doi.org/p3w6 DOI: https://doi.org/10.2174/0929866527666200916141730
Naik AV, Dessai SN, Sellappan K. Antitumour activity of Annona muricata L. leaf methanol extracts against Ehrlich Ascites Carcinoma and Dalton’s Lymphoma Ascites mediated tumours in Swiss albino mice. Libyan J. Med. [Internet]. 2020; 16(1):1846862. doi: https://doi.org/p3w7 DOI: https://doi.org/10.1080/19932820.2020.1846862
Ogbu P, Ugota E, Onwuka R, Ogbu I, Aloke Ch. Effect of acetogenin fraction of Annona muricata leaves on antioxidant status and some indices of benign prostatic hyperplasia in rats. Redox Rep. [Internet]. 2020; 25(1):80–86. doi: https://doi.org/g6wpdb DOI: https://doi.org/10.1080/13510002.2020.1804711
Zubaidi SN, Mohd–Nani H, Ahmad–Kamal MS, Abdul–Qayyum T, Maarof S, Afzan A, Mohmad–Misnan N, Hamezah HS, Baharum SN, Mediani A. Annona muricata: comprehensive review on the ethnomedicinal, phytochemistry, and pharmacological aspects focusing on antidiabetic properties. Life [Internet]. 2023; 13(2):353. doi: https://doi.org/p3w8 DOI: https://doi.org/10.3390/life13020353
Vijay M, Rajitha M, Krishnagaanth M, Jadhav ND, Chigure G, Srivastava A. Controlling deltamethrin–resistant Rhipicephalus microplus with a phytoformulation of Annona muricata and Piper longum. Acta Trop. [Internet]. 2025; 266:107644. doi: https://doi.org/p3w9 DOI: https://doi.org/10.1016/j.actatropica.2025.107644
Castro–Hernández CL, Ayasta–Senmache JG, Santa Cruz– López CY, Carrasco–Solano FA, Moreno–Mantilla M. Efecto antibacteriano del extracto etanólico de Annona muricata sobre microorganismos de importancia clínica. Gac. Méd. Bolívar [Internet]. 2021; 44(1):29-33. doi: https://doi.org/p3xb DOI: https://doi.org/10.47993/gmb.v44i1.219
Coria–Téllez AV, Montalvo–González E, Yahia EM, Obledo– Vázquez EN. Annona muricata: a comprehensive review on its traditional medicinal uses, phytochemicals, pharmacological activities, mechanisms of action and toxicity. Arab. J. Chem. [Internet]. 2018; 11(5):662–691. doi: https://doi.org/gpbc6m DOI: https://doi.org/10.1016/j.arabjc.2016.01.004
Setthawongsin C, Teewasutrakul P, Tangkawattana S, Techangamsuwan S, Rungsipipat A. Conventional–vincristine sulfate vs. modified protocol of vincristine sulfate and L– asparaginase in canine transmissible venereal tumor. Front. Vet. Sci. [Internet]. 2019; 6:300. doi: https://doi.org/p3xd DOI: https://doi.org/10.3389/fvets.2019.00300
Suhail P, Venkatachalam VV, Balasubramanian T, Christapher PV. A review on the in vitro anticancer potentials of acetogenins from Annona muricata Linn.: a potential inducer of Bax–Bak and Caspase-3 related pathways. Indian J. Pharm. Educ. Res. [Internet]. 2024; 58(3Suppl):S693. doi: https://doi.org/p3xf DOI: https://doi.org/10.5530/ijper.58.3s.73
Md Roduan MR, Abd Hamid R, Mohtarrudin N. Modulation of cancer signalling pathway(s) in two–stage mouse skin tumorigenesis by annonacin. BMC Complement. Med. Ther. [Internet]. 2019; 19:238. doi: https://doi.org/p3xg DOI: https://doi.org/10.1186/s12906-019-2650-1
Kariyil BJ, Ayyappan UPT, Gopalakrishnan A, George AJ. Chloroform fraction of methanolic extract of seeds of Annona muricata induce S phase arrest and ROS dependent caspase activated mitochondria–mediated apoptosis in triple–negative breast cancer. Anticancer Agents Med. Chem. [Internet]. 2021; 21(10):1250–1265. doi: https://doi.org/p3xh DOI: https://doi.org/10.2174/1871520620666200918101448
Li RS, Li LY, Zhu XF, Li X, Wang CY, Qiu SJ, Zhou J, Fan J, Hu B, Mu Q Annonaceous acetogenins synergistically inhibit hepatocellular carcinoma with sorafenib. J. Nat. Prod. [Internet]. 2024; 87(1):14-27. doi: https://doi.org/p3xj DOI: https://doi.org/10.1021/acs.jnatprod.3c00667
Grba DN, Blaza JN, Bridges HR, Agip ANA, Yin Z, Murai M, Miyoshi H, Hirst J. Cryo–electron microscopy reveals how acetogenins inhibit mitochondrial respiratory complex I. J. Biol. Chem. [Internet]. 2022; 298(3):101602. doi: https://doi.org/p3xm DOI: https://doi.org/10.1016/j.jbc.2022.101602
Chauhan S, Srivastava V. Evaluation acute toxicity studies of hydro alcoholic extract of soursop leaves (Annona muricata) on brain, liver and kidney of Wister rats. J. Clin. Toxicol. 2024 [cited May 14 2025]; 14(1):556. Available in: https://goo.su/SLzAQS
Zeweil MM, Khafaga AF, Mahmoud SF, Wasef L, Saleh H, Elrehim AMA, Bassuoni NF, Alwaili MA, Saeedi NH, Ghoneim HA. Annona muricata L. extract restores renal function, oxidative stress, immunohistochemical structure, and gene expression of TNF–α, IL–β1, and CYP2E1 in the kidney of DMBA–intoxicated rats. Front. Pharmacol. [Internet]. 2024; 15:1348145. doi: https://doi.org/gt33nw DOI: https://doi.org/10.3389/fphar.2024.1348145
Ferreira–Bulhosa L, Estrela–Lima A, da Silva Solcà M, Diniz Gonçalves GS, Larangeira DF, de Pinho FA, Barrouin–Melo SM. Vincristine and ivermectin combination chemotherapy in dogs with natural transmissible venereal tumor of different cyto– morphological patterns: a prospective outcome evaluation. Anim. Reprod. Sci. [Internet]. 2020; 216:106358. doi: https://doi.org/p3xn DOI: https://doi.org/10.1016/j.anireprosci.2020.106358