Assessment of the acute and subacute toxicity of Algerian Hyoseris radiata L. in the Wistar albino rats model
Abstract
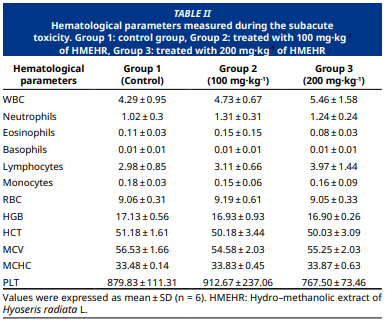
Wild chicory, or Hyoseris radiata L., is indigenous to the Mediterranean region, is a plant used in traditional medicine as a diuretic, blood depurative, and against kidney stones. The present study aimed to assess for the first time the acute and subacute toxicity, to quantify the total amount of polyphenols and flavonoids, and to assess the antioxidant activity of H. radiata collected from Setif, Algeria. The overall amount of flavonoids and polyphenols was quantified spectrophotometrically. The antioxidant activity of the extract was evaluated according to two methods, DPPH and FRAP. The acute toxicity of H. radiata was carried out according to the OECD guideline 423 to determine the median lethal dose LD50 and the subacute toxicity was evaluated according to OECD guideline 407 to assess the possible pathological effects of the extract administered for 28 days by oral route. The results show that the total amount of polyphenols and flavonoids was 132.53 ± 2 µg of GAE·1 mg-1 and 96.11 ± 3.65 µg of QE·1 mg-1 of extract, respectively. The extract shows a good antioxidant potential in both tests. The administered dose (2 g·kg-1 of BW) didn’t produce any changes in general behaviors or mortality, so the LD50 is greater than 2 g·kg-1 of BW. Moreover, the daily administration of the extract with 2 doses, 100 mg·kg-1 and 200 mg·kg-1 didn’t cause any changes in body weight, behavior test, hematological parameters, and organ relative weight. A significant decrease in triglyceride was recorded in both concentrations. Based on the present findings, the extract of H. radiata has no significant toxicity. These findings offer valuable information about the toxicity profile of the traditional medicine plant Hyoseris radiata L.
Downloads
References
Ha AW, Kang HJ, Kim SL, Kim MH, Kim WK. Acute and subacute toxicity evaluation of corn silk extract. Prev. Nutr. Food Sci. [Internet]. 2018; 23(1):70-76. doi: https://doi.org/pzh3x DOI: https://doi.org/10.3746/pnf.2018.23.1.70
Gori L, Firenzuoli F. Pharmacovigilance: Tools in establishing the safety and acceptability of the natural health products— Clinical evaluation. In: Mukherjee PK, editor. Evidence–Based Validation of Herbal Medicine. [Internet]. New Delhi (India): Elsevier; 2015. p. 165–174. doi: https://doi.org/pzh4 DOI: https://doi.org/10.1016/B978-0-12-800874-4.00007-6
Bello I, Bakkouri AS, Tabana YM, Al–Hindi B, Al–Mansoub MA, Mahmud R, Asmawi MZ. Acute and sub–acute toxicity evaluation of the methanolic extract of Alstonia scholaris Stem Bark. Med. Sci. [Internet]. 2016; 4(1):4. doi: https://doi.org/gtn26f DOI: https://doi.org/10.3390/medsci4010004
Brondani JC, Reginato FZ, da Silva Brum E, de Souza Vencato M, Lima Lhamas C, Viana C, da Rocha MI, de Freitas Bauermann L, Manfron MP. Evaluation of acute and subacute toxicity of hydroethanolic extract of Dolichandra unguis–cati L. leaves in rats. J. Ethnopharmacol. [Internet]. 2017; 202:147-153. doi: https://doi.org/f96s7c DOI: https://doi.org/10.1016/j.jep.2017.03.011
Wu Z, Ma Y, Zhao L, Cai S, Cheng G. Acute and subchronic toxicities of the ethanol and hot–water extracts from Chinese sumac (Rhus chinensis Mill.) fruits by oral administration in rats. Food Chem. Toxicol. [Internet]. 2018; 119:14–23. doi: https://doi.org/gd7xg8 DOI: https://doi.org/10.1016/j.fct.2018.06.009
Li Y, Zhuang Y, Tian W, Sun L. In vivo acute and subacute toxicities of phenolic extract from rambutan (Nephelium lappaceum) peels by oral administration. Food Chem. [Internet]. 2020; 320:126618. doi: https://doi.org/gp5cmp DOI: https://doi.org/10.1016/j.foodchem.2020.126618
Organization for Economic Cooperation and Development (OECD). Test No. 423: Acute oral toxicity – Acute toxic class method. OECD Guidel. Test Chem. Section 4. Paris: OECD Publishing; 2002. doi: https://doi.org/bxtb9z
Organization for Economic Cooperation and Development (OECD). Test No. 407: Repeated dose 28-day oral toxicity study in rodents. OECD Guidel. Test Chem. Section 4; Paris: OECD Publishing; 2025. doi: https://doi.org/cv4qr2
Quezel P, Santa S. Nouvelle flore de l’Algérie et des régions désertiques méridionales. Tome II. Editions du Centre National de la Recherche Scientifique, 1963. In: Bourlière F, editor. La Terre et La Vie, Revue d’Histoire Naturelle. 1964; 18(2):238.
Guarrera PM, Savo V. Wild food plants used in traditional vegetable mixtures in Italy. J. Ethnopharmacol. [Internet]. 2016; 185:202–234. doi: https://doi.org/pzqc DOI: https://doi.org/10.1016/j.jep.2016.02.050
Vitiello M, Pecoraro M, De Leo M, Camangi F, Parisi V, Donadio G, Braka A, Franceschelli S, De Tommasi N. Chemical profiling, antioxidant, and anti–inflammatory activities of Hyoseris Radiata L., a plant used in the phyt alimurgic tradition. Antioxidants [Internet]. 2024; 13(1):111. doi: https://doi.org/pzqd DOI: https://doi.org/10.3390/antiox13010111
Hamia C, Guergab A, Rennane N, Birache M, Haddad M, Saidi M, Yousfi M. Influence des solvants sur le contenu en composés phénoliques et l’activité antioxydante des extraits du Rhanterium adpressium. Ann. Sci. Technol. [Internet]. 2014 [cited 2025 Feb. 15]; 6(1):7. Available in: https://goo.su/qtd8 DOI: https://doi.org/10.12816/0010624
Le K, Chiu F, Ng K. Identification and quantification of antioxidants in Fructus lycii. Food Chem. [Internet]. 2007; 105(1):353–363. doi: https://doi.org/cgjj24 DOI: https://doi.org/10.1016/j.foodchem.2006.11.063
Turkoglu A, Duru ME, Mercan N, Kivrak I, Gezer K. Antioxidant and antimicrobial activities of Laetiporus sulphureus (Bull.) Murrill. Food Chem. [Internet]. 2007; 101(1):267–273. doi: https://doi.org/cctm9n DOI: https://doi.org/10.1016/j.foodchem.2006.01.025
Burits M, Bucar F. Antioxidant activity of Nigella sativa essential oil. Phytother. Res. [Internet]. 2000; 14(5):323–328. doi: https://doi.org/b639sn DOI: https://doi.org/10.1002/1099-1573(200008)14:5<323::AID-PTR621>3.0.CO;2-Q
Oyaizu M. Studies on products of browning reaction antioxidative activities of products of browning reaction prepared from glucosamine. Japan. J. Nutr. Diet. [Internet]. 1986; 44(6):307–315. doi: https://doi.org/cp87v4 DOI: https://doi.org/10.5264/eiyogakuzashi.44.307
Tabassum S, Haider S, Ahmad S, Madiha S, Parveen T. Chronic choline supplementation improves cognitive and motor performance via modulating oxidative and neurochemical status in rats. Pharmacol. Biochem. Behav. [Internet]. 2017; 159:90–99. doi: https://doi.org/gbvmnb DOI: https://doi.org/10.1016/j.pbb.2017.05.011
Deacon RMJ. Measuring the strength of mice. J. Vis. Exp. [Internet]. 2013; 76:2610. doi: https://doi.org/pzqj DOI: https://doi.org/10.3791/2610
Martey ON, Armah G, Okine LK. Absence of organ specific toxicity in rats treated with Tonica, an aqueous herbal haematinic preparation. Afr. J. Tradit. Complement. Altern. Med. [Internet]. 2010; 7(3):231-240. doi: https://doi.org/db7kt9 DOI: https://doi.org/10.4314/ajtcam.v7i3.54781
Sicari V, Loizzo MR, Sanches Silva A, Romeo R, Spampinato G, Tundis R, Leporini M, Musarella CM. The effect of blanching on phytochemical content and bioactivity of Hypochaeris and Hyoseris Species (Asteraceae), vegetables traditionally used in Southern Italy. Foods [Internet]. 2020; 10(1):32. doi: https://doi.org/pzqk DOI: https://doi.org/10.3390/foods10010032
Huang D, Ou B, Prior RL. The chemistry behind antioxidant capacity assays. J. Agric. Food Chem. [Internet]. 2005; 53(6):1841–1856. doi: https://doi.org/ck822z DOI: https://doi.org/10.1021/jf030723c
Chaturvedula VSP, Norris A, Miller JS, Ratovoson F, Andriantsiferana R, Rasamison VE, Kingston DG. Cytotoxic Diterpenes from Cassipourea madagascariensis from the Madagascar Rainforest. J. Nat. Prod. [Internet]. 2006; 69(2):287–289. doi: https://doi.org/bm5mgb DOI: https://doi.org/10.1021/np050376w
Asare GA, Addo P, Bugyei K, Gyan B, Adjei S, Otu–Nyarko LS, Wiredu EK, Nyarko A. Acute toxicity studies of aqueous leaf extract of Phyllanthus niruri. Interdiscip. Toxicol. [Internet]. 2011; 4(4):206-210. doi: https://doi.org/pzqm DOI: https://doi.org/10.2478/v10102-011-0031-9
Li X, Luo Y, Wang L, Li Y, Shi Y, Cui Y, Xue M. Acute and subacute toxicity of ethanol extracts from Salvia przewalskii Maxim in rodents. J. Ethnopharmacol. [Internet]. 2010; 131(1):1100-1105. doi: https://doi.org/b7vh75 DOI: https://doi.org/10.1016/j.jep.2010.06.012
Nouioura G, Tourabi M, Tahraoui A, El–Yagoubi K, Maache S, Elfatemi H, Lyoussi B, Derwich EH. Assessment of the acute and subacute toxicity of the aqueous extract of Moroccan Ferula communis fruit in a mouse model. Saudi Pharm. J. [Internet]. 2023; 31(8):101701. doi: https://doi.org/pzqn DOI: https://doi.org/10.1016/j.jsps.2023.101701
Raina P, Chandrasekaran CV, Deepak M, Agarwal A, Ruchika KG. Evaluation of subacute toxicity of methanolic/aqueous preparation of aerial parts of O. sanctum in Wistar rats: Clinical, haematological, biochemical and histopathological studies. J. Ethnopharmacol. [Internet]. 2015; 175:509–517. doi: https://doi.org/f8g5c5 DOI: https://doi.org/10.1016/j.jep.2015.10.015