Assessment of semen quality in freshwater mussel Unio elongatulus eucirrus: analysis of sperm motility, morphometry, and pH
Abstract
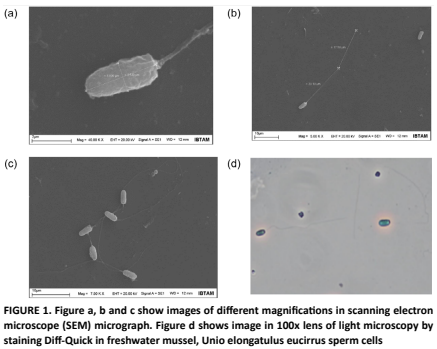
The freshwater mussel (Unio elongatulus eucirrus), native to the Euphrates River basin in Türkiye, holds little economic value; however, it plays an important ecological role in natural aquatic ecosystems. Consequently, understanding its biology particularly its reproductive traits, such as semen quality, sperm motility, and morphology is of scientific interest. This study aimed to investigate several reproductive parameters in 10 male specimens of this species, including sperm concentration, seminal pH, motility characteristics, and sperm morphometry. Sperm kinematics were assessed using a computer-assisted sperm analysis (CASA) system. The results showed the following sperm velocity values: straight- line velocity (VSL: 40.75 ± 6.01 μm/s), curvilinear velocity (VCL: 103.00 ± 2.62 μm/s), and average path velocity (VAP: 54.24 ± 6.75 μm/s). Sperm morphometry, analyzed via scanning electron microscopy (SEM), revealed a head length of 3.90 ± 0.11 μm, head width of 1.70 ± 0.17 μm, and tail length of 37.64 ± 0.45 μm. Regarding the physicochemical parameters, the seminal pH was 6.25 ± 0.26, and sperm concentration was 15.48 ± 0.53 × 109 cells/mL. These results indicate that the morphological and kinematic characteristics of freshwater mussel (Unio elongatulus eucirrus) spermatozoa are quite like those observed in other mussel and fish species. However, it was determined that spermatozoa are easily activated upon contact with water.
Downloads
References
Vaughn CC. Ecosystem services provided by freshwater mussels. Hydrobiol. [Internet]. 2018; 810:15–27. doi: https://doi.org/gcmfpr DOI: https://doi.org/10.1007/s10750-017-3139-x
Howard JK, Cuffey KM. The functional role of native freshwater mussels in the fluvial benthic environment. Freshw. Biol. [Internet]. 2006; 51(3):460–474. doi: https://doi.org/cxcdbd DOI: https://doi.org/10.1111/j.1365-2427.2005.01507.x
Strayer D. Freshwater Mussel Ecology. University of California Press; 2008. https://doi.org/pk3 DOI: https://doi.org/10.1525/california/9780520255265.001.0001
Çetinkaya O. A Freshwater Mussel Species Unio stevenianus Krynicki 1837 (Mollusca: Bivalvia: Unionidae) from the River Karasu Flowing into Lake Van, Turkey. Turkish J Zool. [Internet]. 1996[cited December 18, 2024]; 20(2):163-173. Available in: https://goo.su/PWwTPq DOI: https://doi.org/10.55730/1300-0179.3028
Billard R, Cosson J, Crim L, Suquet M. Sperm physiology and quality. In: Bromage NR RR, editor. Broodstock Manag. egg larval Qual., Cambridge: Blackwell Science; 1995.
Rurangwa E, Kime DE, Ollevier F, Nash JP. The measurement of sperm motility and factors affecting sperm quality in cultured fish. Aquac. [Internet]. 2004; 234(1-4):1–28. doi: https://doi.org/d65ndj DOI: https://doi.org/10.1016/j.aquaculture.2003.12.006
Lahnsteiner F, Berger B, Weismann T, Patzner RA. Determination of semen quality of the rainbow trout, Oncorhynchus mykiss, by sperm motility, seminal plasma parameters, and spermatozoa metabolism. Aquac. [Internet].1998; 163(1-2):163–181. doi: https://doi.org/dx5vzv DOI: https://doi.org/10.1016/S0044-8486(98)00243-9
Fauvel C, Suquet M, Cosson J. Evaluation of fish sperm quality. J. Appl. Ichthyol. [Internet].2010; 26:636–643. doi: https://doi.org/dp5sm3 DOI: https://doi.org/10.1111/j.1439-0426.2010.01529.x
Amann RP, Waberski D. Computer-assisted sperm analysis (CASA): Capabilities and potential developments. Theriogenology. [Internet]. 2014; 81(1):5-17.e3. doi: https://doi.org/ggxqpw DOI: https://doi.org/10.1016/j.theriogenology.2013.09.004
Hadi-Alavi SM, Matsumura N, Shiba K, Itoh N, Takahashi KG, Inaba K, Osada M. Roles of extracellular ions and pH in 5-HT-induced sperm motility in marine bivalve. Reprod. [Internet]. 2014; 147(3):331–345. doi: https://doi.org/pk3v DOI: https://doi.org/10.1530/REP-13-0418
Waller DL, Lasee BA. External Morphology of Spermatozoa and Spermatozeugmata of the Freshwater Mussel Truncilla truncata (Mollusca: Bivalvia: Unionidae). Am. Midl. Nat. [Internet]. 1997; 138(1):220. doi: https://doi.org/bgbdk6 DOI: https://doi.org/10.2307/2426669
Healy JM. Spermatozoon ultrastructure in the trigonioid bivalve Neotrigonia margaritacea Lamarck (Mollusca): Comparison with other bivalves, especially Trigonioida and Unionoida. Helgoländer Meeresuntersuchungen [Internet]. 1996; 50:259–264. doi: https://doi.org/cj4gv3 DOI: https://doi.org/10.1007/BF02367155
Misamore M, Silverman H, Lynn JW. Analysis of fertilization and polyspermy in serotonin-spawned eggs of the zebra mussel, Dreissena polymorpha. Mol. Reprod. Dev. [Internet]. 1996; 43(2):205–216. doi: https://doi.org/d8k4d6 DOI: https://doi.org/10.1002/(SICI)1098-2795(199602)43:2<205::AID-MRD10>3.3.CO;2-5
Ram JL, Fong PP, Garton DW. Physiological Aspects of Zebra Mussel Reproduction: Maturation, Spawning, and Fertilization. Am. Zool. [Internet]. 1996; 36(3):326–338. doi: https://doi.org/fp7jj6 DOI: https://doi.org/10.1093/icb/36.3.326
Demirsoy A. Basic Rules of Life, Invertebrates= Invertebrata (except insects) (in Turkish). Vol. Cilt I Kısım I. Ankara: Meteksan; 1998.
Özgür ME, Okumuş F, Kocamaz AF. Novel Computer Assisted Sperm Analyzer for Assessment of Spermatozoa Motility in Fish; BASA-Sperm Aqua. El-Cezeri J. Eng. Sci. [Internet]. 2019; 6(1):208-219. doi: https://doi.org/pk3w DOI: https://doi.org/10.31202/ecjse.486342
Nichols ZG, Zadmajid V, Dalal V, Stoeckel J, Wayman W, Butts IAE. Reproductive aspects of freshwater unionid mussel sperm: Seasonal dynamics, male-to male variability, and cell quantification. Anim. Reprod. Sci. [Internet]. 2021; 230:106768. doi: https://doi.org/gkkrgp DOI: https://doi.org/10.1016/j.anireprosci.2021.106768
Wei Q, Li P, Psenicka M, Hadi-Alavi SM, Shen L, Liu J, Peknicova J, Linhart O. Ultrastructure and morphology of spermatozoa in Chinese sturgeon (Acipenser sinensis Gray 1835) using scanning and transmission electron microscopy. Theriogenology. [Internet]. 2007; 67(7):1269–1278. doi: https://doi.org/d7jfv5 DOI: https://doi.org/10.1016/j.theriogenology.2007.02.003
Haszprunar G, Jamieson BGM. Fish Evolution and Systematics: Evidence from Spermatozoa. With a survey of lophophorate, echinoderm and protochordate sperm and an account of gamete cryopreservation. J. Evol. Biol. [Internet]. 1992; 5(4):721–723. doi: https://doi.org/d5vpvj DOI: https://doi.org/10.1046/j.1420-9101.1992.5040721.x
Lahnsteiner F, Patzner R. Sperm morphology and ultrastructure in fish. In: Alavi SMH, Cosson J, Coward K, Rafiee G, editors., Oxford: Alpha Science International Ltd.; 2008, p. 1–61.
Butts IAE, Ward MAR, Litvak MK, Pitcher TE, Alavi SMH, Trippel EA, Rideout RM. Automated sperm head morphology analyzer for open-source software. Theriogenology. [Internet]. 2011; 76(9):1756-1761.e3. doi: https://doi.org/fccgws DOI: https://doi.org/10.1016/j.theriogenology.2011.06.019
Hadi-Alavi SM, Hatef A, Psenicka M, Kaspar V, Boryshpolets S, Dzyuba B, Cosson J, Bondarenko V, Rodina M, Gela D, Linhart O. Sperm biology and control of reproduction in sturgeon: (II) sperm morphology, acrosome reaction, motility and cryopreservation. Rev. Fish Biol. Fish [Internet]. 2012; 22:861–886. doi: https://doi.org/g7r5xn DOI: https://doi.org/10.1007/s11160-012-9270-x
Hatef A, Alavi SMH, Rodina M, Linhart O. Morphology and fine structure of the Russian sturgeon, Acipenser gueldenstaedtii (Acipenseridae, Chondrostei) spermatozoa. J. Appl. Ichthyol. [Internet]. 2012; 28:978– 983. doi: https://doi.org/f4d7p5 DOI: https://doi.org/10.1111/jai.12056
Ciereszko A, Dabrowski K, Piros B, Kwasnik M, Glogowski J. Characterization of zebra mussel (Dreissena polymorpha) sperm motility: Duration of movement, effects of cations, pH and gossypol. Hydrobiology. [Internet]. 2001; 452:225–232. doi: https://doi.org/cm98v3 DOI: https://doi.org/10.1023/A:1011922820657
Fitzpatrick JL, Nadella S, Bucking C, Balshine S, Wood CM. The relative sensitivity of sperm, eggs and embryos to copper in the blue mussel (Mytilus trossulus). Comp. Biochem. Physiol. C Toxicol. Pharmacol. [Internet]. 2008; 147(4):441–449. doi: https://doi.org/bbcwfh DOI: https://doi.org/10.1016/j.cbpc.2008.01.012
Abdelsaleheen O, Taskinen J, Kortet R. Reproductive cycle, fecundity and gro)wth of the freshwater mussel Unio tumidus (Bivalvia: Unionidae) from Lake Viinijärvi, Finland. J. Molluscan Stud. [Internet]. 2024; 90(3):3. doi: https://doi.org/pk3z DOI: https://doi.org/10.1093/mollus/eyae024
Lynn JW. The ultrastructure of the sperm and motile spermatozeugmata released from the freshwater mussel Anodonta grandis (Mollusca, Bivalvia, Unionidae). Can. J. Zool. [Internet]. 1994; 72(8):1452–1461. doi: https://doi.org/ftnsjw DOI: https://doi.org/10.1139/z94-192
Peredo S, Parada E. Gonadal organization and gametogenesis in the fresh-water mussel Diplodon chilensis chilensis (Mollusca, Bivalvia). The Veliger. 1984[cited Nov 23, 2024]; 27(2):126-133. Available in: https://goo.su/PabCb
Chen J, Deng Z, Wei H, Zhao W, Chen M, Yu G, Sun J, Yu D, Li Y, Wang Y, Bai L. Spermatozoa morphology and embryo development of four species of bivalves from Beibu Gulf. Turk. J. Fish. Aquat. Sci. [Internet]. 2021; 21(2):51-61. doi: https://doi.org/gm2rhb DOI: https://doi.org/10.4194/1303-2712-v21_2_01
Yurchenko OV, Borzykh OG, Kalachev AV. Ultrastructural aspects of spermatogenesis in Calyptogena pacifica Dall 1891 (Vesicomyidae; Bivalvia). J. Morphol. [Internet]. 2021; 282(1):146-159. doi: https://doi.org/pk33 DOI: https://doi.org/10.1002/jmor.21292
Healy JM, Mikkelsen PM, Bieler R. Sperm ultrastructure in the ocean quahog Arctica islandica (Arcticidae) and Neotrapezium sublaevigatum (Trapezidae), with a discussion of relationships within the Arcticoidea and with other Euheterodonta (Bivalvia). J. Molluscan Stud. [Internet]. 2020; 86(3):173–185. doi: https://doi.org/pk34 DOI: https://doi.org/10.1093/mollus/eyaa002