Evaluación de los niveles de Nesfatina-1 en ovejas con toxemia gestacional
Resumen
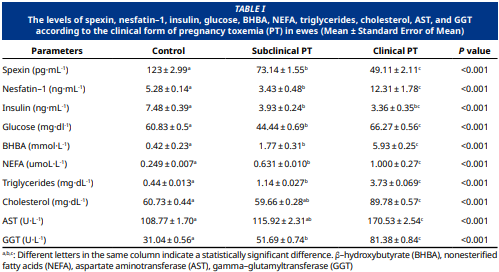
El objetivo de este estudio fue investigar los efectos de la toxemia gestacional (TP) en los niveles de nesfatina–1 en ovejas y examinar su relación con la espexina, otro biomarcador involucrado en el metabolismo energético. El estudio se realizó en 45 ovejas Awassi que habían parido al menos una vez y tenían entre 120 y 150 días de gestación. Las ovejas se dividieron en tres grupos. El grupo control (Grupo 1, n=15) consistió en ovejas sanas con niveles de BHBA (ácido β–hidroxibutírico) < 0,8 mmol·L-1, el grupo PT subclínico (Grupo 2, n=15) incluyó ovejas con niveles de BHBA entre 0,8 y 2,5 mmol·L-1, y el grupo PT clínico (Grupo 3, n=15) consistió en ovejas con niveles de BHBA > 2,5 mmol·L-1 (rango de 2,6 a 7,10 mmol·L-1). Los niveles séricos de nesfatina–1 y espexina se midieron utilizando un kit comercial. Los niveles de espexina fueron significativamente menores en los grupos de PT subclínico y clínico en comparación con el grupo control (P<0,001). Los niveles de nesfatina–1 fueron menores en el grupo de PT subclínico, pero mayores en el grupo de PT clínico en comparación con el control (P<0,001). Los niveles de triglicéridos y colesterol fueron significativamente mayores en los grupos de PT subclínico y clínico en comparación con el control (P<0,001). Los niveles de aspartato aminotransferasa (AST) y gama glutamil transferasa (GGT) también fueron significativamente elevados en los grupos de PT subclínico y clínico (P<0,001). El análisis de correlación reveló las siguientes relaciones: los niveles de espexina mostraron una correlación positiva significativa con la insulina (P<0,001). Los niveles de espexina se correlacionaron negativamente con nesfatina–1, BHBA, (ácidos grasos no esterificados) NEFA, triglicéridos, colesterol, AST y GGT (P<0,001). Los niveles de nesfatina–1 se correlacionaron positivamente con la glucosa, BHBA, NEFA, triglicéridos, colesterol, AST y GGT, mientras que mostraron una correlación negativa significativa con la insulina (P<0,001). En conclusión, las neuropéptidos nesfatina–1 y espexina podrían servir como nuevos biomarcadores para el diagnóstico de las formas clínicas y subclínicas de TP en ovejas.
Descargas
Citas
Rook JS. Pregnancy toxemia of ewes, does and beef cows.Vet. Clin. N. Am. Food A. [Internet]. 2000; 16(2):293–317. doi: https://doi.org/njpx DOI: https://doi.org/10.1016/S0749-0720(15)30107-9
Souto RJC, Afonso JAB, Mendonça CL, Carvalho CCD, Filho S, Cajueiro FP, Lima EHF, Soares PC. Biochemical, electrolytic and hormonal findings in goats afected with pregnancy toxaemia. Pesq. Vet. [Internet]. 2013; 33(10):1174-1182. doi: https://doi.org/pzwx DOI: https://doi.org/10.1590/S0100-736X2013001000002
Lacetera N, Bernabucci U, Ronchi B, Nardone A. Efects of subclinical pregnancy toxemia on immune responses in sheep. Am. J. Vet. Res. [Internet]. 2001; 62(7):1020-1024. doi: https://doi.org/b4bffg DOI: https://doi.org/10.2460/ajvr.2001.62.1020
Soares GSL, Ribeiro ACS, Paula JFC, Souto RJC, Oliveira Filho EF, Soares PC, Mendonça CL, Afonso JAB. Cardiac biomarkers and blood metabolites in cows with clinical ketosis. Semina Ciênc. Agrár. [Internet]. 2019; 40(6 Supl. 3):3525–3540. doi: https://doi.org/pzwz DOI: https://doi.org/10.5433/1679-0359.2019v40n6Supl3p3525
Mirabeau O, Perlas E, Severini C, Audero E, Gascuel O, Possenti R, Birney E, Rosenthal N, Gross C. Identification of novel peptide hormones in the human proteome by hidden Markov model screening. Genome Res. [Internet]. 2007; 17(3):320–327. doi: https://doi.org/bzkfsx DOI: https://doi.org/10.1101/gr.5755407
Gu L, Ma Y, Gu M, Zhang Y, Yan S, Li N, Wang Y, Ding X, Yin J, Fan N, Peng Y. Spexin peptide is expressed in human endocrine and epithelial tissues and reduced after glucose load in type 2 diabetes. Peptides [Internet]. 2015; 71:232–239. doi: https://doi.org/f7r6cz DOI: https://doi.org/10.1016/j.peptides.2015.07.018
Karaca A, Bakar Ates F, Ersoz Gulcelik N. Decreased spexin levels in patients with type 1 and type 2 diabetes. Med. Princ. Pract. [Internet]. 2019; 27(6):549–554. doi: https://doi.org/pzw2 DOI: https://doi.org/10.1159/000493482
Öztürk Özkan G. Efects of nesfatin–1 on food intake and hyperglycemia. J. Am. Coll. Nutr. [Internet]. 2020; 39(4):345–351. doi: https://doi.org/pzw3 DOI: https://doi.org/10.1080/07315724.2019.1646678
Alotibi MN, Alnoury AM, Alhozali AM. Serum nesfatin–1 and galanin concentrations in the adult with metabolic syndrome. Relationships to insulin resistance and obesity. Saudi Med. [Internet]. 2019; 40(1):19–25. doi: https://doi.org/pzw4 DOI: https://doi.org/10.15537/smj.2019.1.22825
Chen X, Shu X, Cong ZK, Jiang ZY, Jiang H. Nesfatin–1 acts on the dopaminergic reward pathway to inhibit food intake. Neuropeptides [Internet]. 2015; 53:45-50. doi: https://doi.org/f7wmnz DOI: https://doi.org/10.1016/j.npep.2015.07.004
Andrews A. Pregnancy toxaemia in the ewe. In Pract. [Internet]. 1997; 19(6):306-314. doi: https://doi.org/cqddsn DOI: https://doi.org/10.1136/inpract.19.6.306
Iqbal R, Beigh SA, Mir AQ, Shaheen M, Hussain SA, Nisar M, Dar AA. Evaluation of metabolic and oxidative profile in ovine pregnancy toxemia and to determine their association with diagnosis and prognosis of disease. Trop. Anim. Health Prod. [Internet]. 2022; 54:338. doi: https://doi.org/g5ggb8 DOI: https://doi.org/10.1007/s11250-022-03339-9
Kolakowski LF, O’Neill GP, Howard AD, Broussard SR, Sullivan KA, Feighner SD, , Sawzdargo M, Nguyen T, Kargman S, Shiao LL, Hreniuk DL, Tan CP, Evans J, Abramovitz M, Chateauneuf A, Coulombe N, Ng G, Johnson MP, Tharian A, Khoshbouei A, George SR, Smith RG, O’Dowd BF. Molecular characterization and expression of cloned human galanin receptors GALR2 and GALR3. J. Neurochem. [Internet]. 1998; 71(6):2239–2251. doi: https://doi.org/d887kh DOI: https://doi.org/10.1046/j.1471-4159.1998.71062239.x
Walewski JL, Ge F, Lobdell IV, Levin N, Schwartz GJ, Vasselli JR, Pomp A, Dakin G, Berk PD. Spexin is a novel human peptide that reduces adipocyte uptake of long chain fatty acids and causes weight loss in rodents with diet–induced obesity. Obesity [Internet]. 2014; 22(7):1643–1652. doi: https://doi.org/f58whj DOI: https://doi.org/10.1002/oby.20725
Gambaro SE, Zubiría MG, Giordano AP, Portales AE, Alzamendi A, Rumbo M, Giovambattista A. Spexin improves adipose tissue inflammation and macrophage recruitment in obese mice. Biochim. Biophys. Acta, Mol. Cell Biol. Lipids [Internet]. 2020; 1865(7):158700. doi: https://doi.org/g672b2 DOI: https://doi.org/10.1016/j.bbalip.2020.158700
Mikuła R, Pruszyńska–Oszmałek E, Pszczola M, Rząsińska J, Sassek M, Nowak KW, Nogowski L, Kołodziejski PA. Changes in metabolic and hormonal profiles during transition period in dairy cattle–the role of spexin. BMC Vet. Res. [Internet]. 2021; 17:359. doi: https://doi.org/g9dzxd DOI: https://doi.org/10.1186/s12917-021-03069-4
Uzti̇ mür M, Ünal CN. Evaluation of pregnancy toxemia in goats: Metabolic profile, hormonal findings, and redox balance. Small Rumin. Res. [Internet]. 2024; 241:107385. doi: https://doi.org/pzw6 DOI: https://doi.org/10.1016/j.smallrumres.2024.107385
Shimizu H, Oh–i S, Hashimoto K, Nakata M, Yamamoto S, Yoshida N, Eguchi H, Kato I, Inoue K, Satoh T, Okada S, Yamada M, Yada T, Mori M. Peripheral administration of nesfatin–1 reduces food intake in mice: the leptin–independent mechanism. Endocrinol. [Internet]. 2009; 150(2):662-671. doi: https://doi.org/d9tt7g DOI: https://doi.org/10.1210/en.2008-0598
Su Y, Zhang J, Tang Y, Bi F, Liu JN. The novel function of nesfatin–1: anti–hyperglycemia. Biochem. Biophys. Res. Commun. [Internet]. 2010; 391(1):1039-1042. doi: https://doi.org/b363pz DOI: https://doi.org/10.1016/j.bbrc.2009.12.014
Ogiso K, Asakawa A, Amitani H, Nakahara T, Ushikai M, Haruta I, Koyama KI, Amitani M, Harada T, Yasuhara D, Inui Plasma nesfatin–1 concentrations in restricting–type anorexia nervosa. Peptides [Internet]. 2011; 32(1):150-153. doi: https://doi.org/cc4xhs DOI: https://doi.org/10.1016/j.peptides.2010.10.004
Zhang Z, Li L, Yang M, Liu H, Boden G, Yang G. Increased plasma levels of nesfatin–1 in patients with newly diagnosed type 2 diabetes mellitus. Exp. Clin. Endocrinol. Diabetes [Internet]. 2012; 120(2):91-95. doi: https://doi.org/fscsqc DOI: https://doi.org/10.1055/s-0031-1286339
Tan BK, Hallschmid M, Kern W, Lehnert H, Randeva HS. Decreased cerebrospinal fluid/ plasma ratio of the novel satiety molecule, nesfatin–1/NUCB-2, in obese humans: Evidence of nesfatin–1/NUCB-2 resistance and implications for obesity treatment. J. Clin. Endocrinol. Metab. [Internet]. 2011; 96(4):669-673. doi: https://doi.org/fp35cx DOI: https://doi.org/10.1210/jc.2010-1782
Yosten GL. Novel neuropeptides in the control of food intake: neuronostatin and nesfatin–1. Vitam. Horm. [Internet]. 2013; 92:1-25. doi: https://doi.org/pzxx DOI: https://doi.org/10.1016/B978-0-12-410473-0.00001-5
Başar O, Akbal E, Köklü S, Koçak E, Tuna Y, Ekiz F, Gültuna S, Meriç Yilmaz F, Aydoğan T. A novel appetite peptide, nesfatin–1 in patients with non–alcoholic fatty liver disease. Scand. J. Clin. Lab. Invest. [Internet]. 2012; 72(6):479-483. doi: https://doi.org/g7rv2f DOI: https://doi.org/10.3109/00365513.2012.699097
Li Z, Gao L, Tang H, Yin Y, Xiang X, Li Y. Zhao J, Mulholland M, Zhang W. Peripheral effects of nesfatin–1 on glucose homeostasis. PloS One. [Internet]. 2013; 8(8):e71513. doi: https://doi.org/f5d2sk DOI: https://doi.org/10.1371/journal.pone.0071513
Yang M, Zhang Z, Wang C, Li K, Li S, Boden G, Li L, Yang G. Nesfatin–1 action in the brain increases insulin sensitivity through Akt/AMPK/TORC2 pathway in diet–induced insulin resistance. Diabetes. [Internet]. 2012; 61(8):1959-1968. doi: https://doi.org/f35rpk DOI: https://doi.org/10.2337/db11-1755
Nakata M, Manaka K, Yamamoto S, Mori M, Yada T. Nesfatin–1 enhances glucose–induced insulin secretion by promoting Ca2+ influx through L–type channels in mouse islet β–cells. Endocr. J. [Internet]. 2011; 58(4):305-313. doi: https://doi.org/dtrc74 DOI: https://doi.org/10.1507/endocrj.K11E-056
Aslan M, Celik O, Celik N, Turkcuoglu I, Yilmaz E, Karaer A, Simsek Y, Celik E, Aydin, S. Cord blood nesfatin–1 and apelin-36 levels in gestational diabetes mellitus. Endocrine [Internet]. 2012; 41:424-429. doi: https://doi.org/fzdrq9 DOI: https://doi.org/10.1007/s12020-011-9577-8
Bogardus C. Metabolic abnormalities in the development of non–insulin dependent diabetes mellitus. In: LeRoith D, Taylor SI, Olefsky JM, editors. Diabetes Mellitus: A fundamental and clinical text. 3rd ed. Philadelphia (USA): Lippincott–Raven. 1996; p. 459-467.
Herpertz S, Wagener R, Albus C, Kocnar M, Wagner R, Best F, Schleppinghoff BS, Filz HP, Förster K, Thomas W, Mann K, Köhle K, Senf W. Diabetes mellitus and eating disorders: a multicenter study on the comorbidity of the two diseases. J. Psychosom. Res. [Internet]. 1998; 44(3-4):503-515. doi: https://doi.org/dtdd2q DOI: https://doi.org/10.1016/S0022-3999(97)00274-2
Duehlmeier R, Fluegge I, Schwert B, Ganter M. Insulin sensitivity during late gestation in ewes affected by pregnancy toxaemia and in ewes with high and low susceptibility to this disorder. J. Vet. Intern. Med. [Internet]. 2013; 27:359–366. doi: https://doi.org/f4rb2w DOI: https://doi.org/10.1111/jvim.12035
Oh–I S, Shimizu H, Satoh T, Okada S, Adachi S, Inoue K, Eguchi H, Yamamoto M, Imaki T, Hashimoto K, Tsuchiya T, Monden T, Horiguchi K, Yamada M, Mori M. Identification of nesfatin–1 as a satiety molecule in the hypothalamus. Nature [Internet]. 2006; 443(7112):709-712. doi: https://doi.org/bzkh96 DOI: https://doi.org/10.1038/nature05162
Liu Y, Chen X, Qu Y, Song L, Lin Q, Li M, Su K, Li Y, Dong, J. Central nesfatin–1 activates lipid mobilization in adipose tissue and fatty acid oxidation in muscle via the sympathetic nervous system. BioFactors. [Internet]. 2020; 46(3):454-464. doi: https://doi.org/pzz6 DOI: https://doi.org/10.1002/biof.1600
Dong J, Xu H, Xu H, Wang PF, Cai GJ, Song HF, Wang CC, Dong ZT, Ju YJ, Jiang ZY. Nesfatin–1 stimulates fatty–acid oxidation by activating AMP–activated protein kinase in STZ– induced type 2 diabetic mice. PLoS One. [Internet]. 2013; 8(12):83397. doi: https://doi.org/pzz7 DOI: https://doi.org/10.1371/journal.pone.0083397
Pietrocola F, Galluzzi L, Bravo–San Pedro JM, Madeo F, Kroemer G. Acetyl coenzyme A: A central metabolite and second messenger. Cell. Metab. [Internet]. 2015; 21(6):805– 821. doi: https://doi.org/f7d239 DOI: https://doi.org/10.1016/j.cmet.2015.05.014
Watanabe M, Tozzi R, Risi R, Tuccinardi D, Mariani S, Basciani S, Spera G, Lubrano C, Gnessi L. Beneficial effects of the ketogenic diet on nonalcoholic fatty liver disease: a comprehensive review of the literature. Obes. Rev. [Internet]. 2020; 21(8):e13024. doi: https://doi.org/gpnmrs DOI: https://doi.org/10.1111/obr.13024
Bradshaw PC. Acetyl–CoA metabolism and histone acetylation in the regulation of aging and lifespan. Antioxidants [Internet]. 2021; 10(4):572. doi: https://doi.org/gnh2xt DOI: https://doi.org/10.3390/antiox10040572
Tagaya Y, Osaki A, Miura A, Okada S, Ohshima K, Hashimoto K, Yamada M, Satoh T, Shimizu H, Mori M. Secreted nucleobindin-2 inhibits 3T3-L1 adipocyte differentiation. Protein Pept. Lett. [Internet]. 2012; 19(9):997-1004. doi: https://doi.org/f35tz6 DOI: https://doi.org/10.2174/092986612802084546
Kolodziejski PA, Pruszynska Oszmalek E, Micker M, Skrzypski M, Wojciechowicz T, Szwarckopf P, Skieresz Szewczyk K, Nowak KW, Strowski MZ. Spexin: A novel regulator of adipogenesis and fat tissue metabolism. Biochim. Biophys. Acta, Mol. Cell. Biol. Lipids [Internet]. 2018; 1863(10):1228- 1236. doi: https://doi.org/gfdwwt DOI: https://doi.org/10.1016/j.bbalip.2018.08.001